Intro
Explore the Periodic Tables metals, nonmetals, and metalloids, understanding their properties, classifications, and differences, including alkali metals, transition metals, and semimetals.
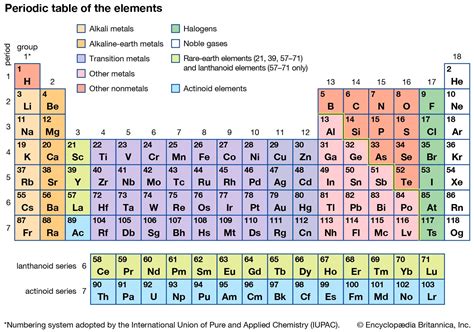
The periodic table is a fundamental tool in chemistry, allowing us to organize and understand the properties of elements. It is divided into three main categories: metals, nonmetals, and metalloids. Understanding the differences between these categories is crucial for understanding the behavior of elements and their potential applications.
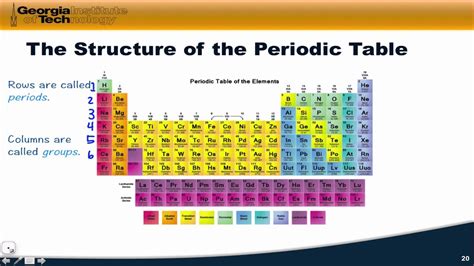
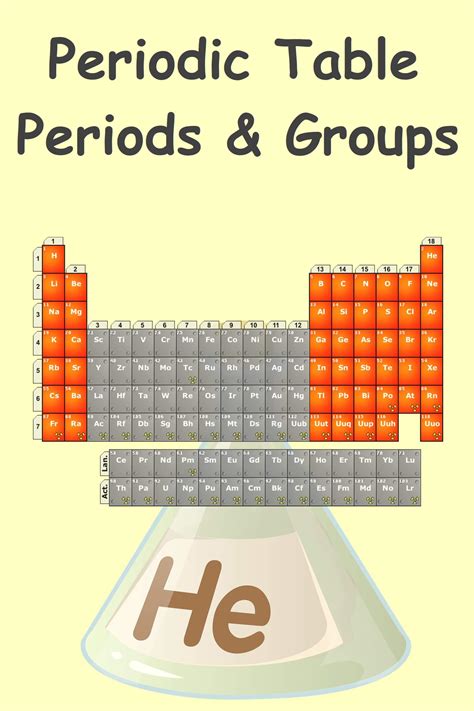
The periodic table is a tabular display of the known chemical elements, organized by their atomic number (number of protons in the nucleus), electron configuration, and recurring chemical properties. The elements are listed in order of increasing atomic number and are grouped into rows called periods and columns called groups or families. The periodic table provides a framework for understanding the relationships between elements and their properties, making it an essential tool for chemists and other scientists.
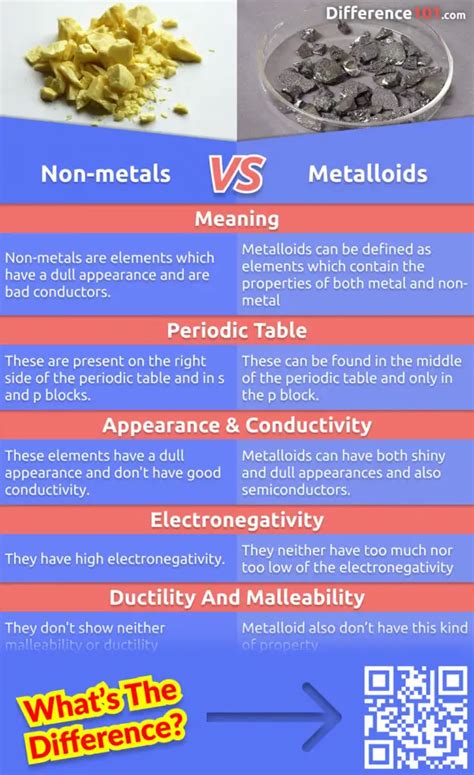
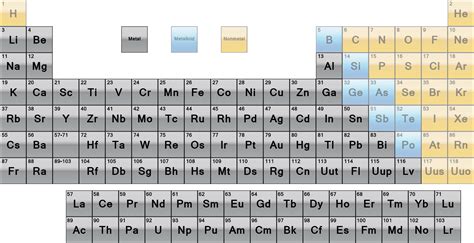
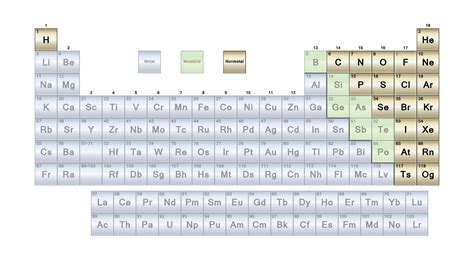
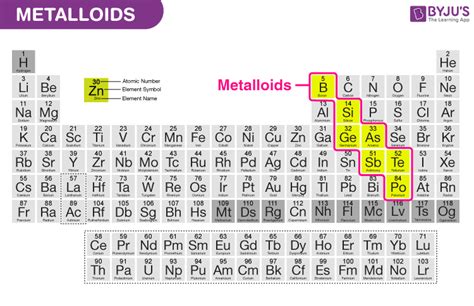
Metals, nonmetals, and metalloids are the three main categories of elements in the periodic table. Metals are typically shiny, malleable, and good conductors of electricity. They are usually found on the left side and center of the periodic table. Nonmetals, on the other hand, are typically dull, brittle, and poor conductors of electricity. They are usually found on the right side of the periodic table. Metalloids are elements that exhibit some properties of metals and some properties of nonmetals. They are usually found on the border between metals and nonmetals in the periodic table.
Introduction to Metals

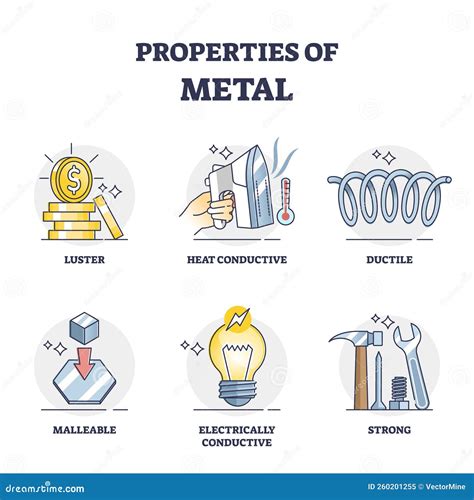
Properties of Metals
Metals have several distinct properties that set them apart from nonmetals and metalloids. Some of the key properties of metals include: * Malleability: Metals can be pounded into thin sheets or drawn into wires without breaking. * Conductivity: Metals are good conductors of electricity and heat. * Luster: Metals are typically shiny and reflective. * Ductility: Metals can be stretched into thin wires without breaking. * High melting and boiling points: Metals tend to have high melting and boiling points, which makes them useful for high-temperature applications.Introduction to Nonmetals
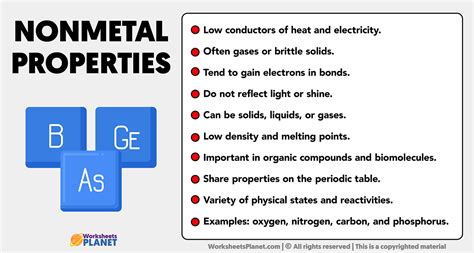
Properties of Nonmetals
Nonmetals have several distinct properties that set them apart from metals and metalloids. Some of the key properties of nonmetals include: * Brittleness: Nonmetals are typically brittle and prone to breaking. * Poor conductivity: Nonmetals are poor conductors of electricity and heat. * Dullness: Nonmetals are typically dull and non-reflective. * Low melting and boiling points: Nonmetals tend to have low melting and boiling points, which makes them useful for low-temperature applications. * High electronegativity: Nonmetals tend to have high electronegativity, which means they tend to attract electrons towards themselves.Introduction to Metalloids
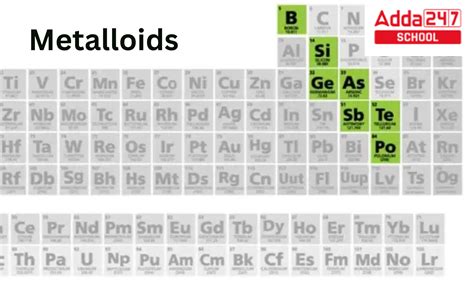
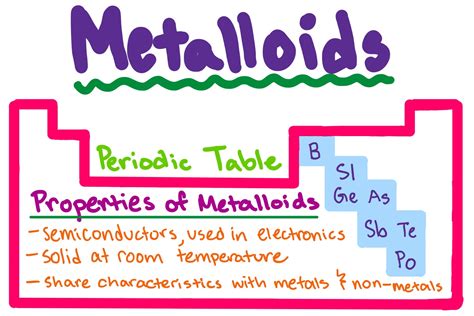
Properties of Metalloids
Metalloids have several distinct properties that set them apart from metals and nonmetals. Some of the key properties of metalloids include: * Intermediate conductivity: Metalloids are intermediate conductors of electricity and heat. * Semi-luster: Metalloids are typically semi-lustrous and partially reflective. * Semi-ductility: Metalloids can be stretched into thin wires, but they tend to be more brittle than metals. * Intermediate melting and boiling points: Metalloids tend to have intermediate melting and boiling points, which makes them useful for a wide range of applications. * Intermediate electronegativity: Metalloids tend to have intermediate electronegativity, which means they tend to attract electrons towards themselves, but not as strongly as nonmetals.Applications of Metals, Nonmetals, and Metalloids

Gallery of Periodic Table Elements
Periodic Table Elements Image Gallery

Frequently Asked Questions
What is the difference between metals and nonmetals?
+Metals are typically shiny, malleable, and good conductors of electricity, while nonmetals are typically dull, brittle, and poor conductors of electricity.
What are metalloids, and where are they found in the periodic table?
+Metalloids are elements that exhibit some properties of metals and some properties of nonmetals. They are typically found on the border between metals and nonmetals in the periodic table.
What are some common applications of metals, nonmetals, and metalloids?
+Metals, nonmetals, and metalloids have a wide range of applications in various fields, including construction, electronics, biology, and medicine. Some common examples include construction materials, electronics, biological molecules, and medical implants.
In summary, the periodic table is a powerful tool for understanding the properties of elements and their potential applications. Metals, nonmetals, and metalloids are the three main categories of elements in the periodic table, each with distinct properties and applications. By understanding the differences between these categories, we can better appreciate the diversity of elements and their role in shaping our world. We encourage you to share your thoughts on the periodic table and its applications in the comments below, and to explore further resources on this topic to deepen your understanding of the elements and their properties.
