Intro
Create molecular models with a VSEPR chart printable template, utilizing electron geometry and polarity to predict shapes, bond angles, and molecular structures.
The VSEPR (Valence Shell Electron Pair Repulsion) theory is a crucial concept in chemistry that helps predict the shape of molecules. It states that electron pairs in the valence shell of an atom repel each other and arrange themselves to minimize repulsion. This theory is essential for understanding the structure and properties of molecules. In this article, we will delve into the world of VSEPR charts, exploring their importance, benefits, and how to use them effectively.
Understanding the VSEPR theory is vital for chemists, researchers, and students alike. It provides a framework for predicting the shape of molecules, which is crucial for understanding their chemical and physical properties. The VSEPR theory is based on the idea that electron pairs in the valence shell of an atom repel each other due to their negative charge. This repulsion causes the electron pairs to arrange themselves in a way that minimizes repulsion, resulting in a specific molecular shape.
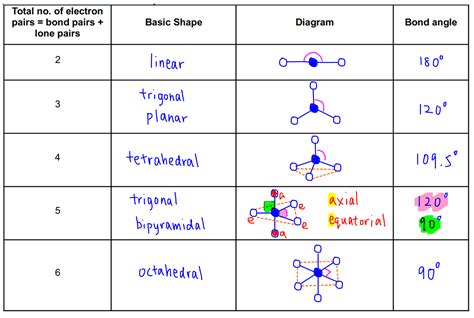
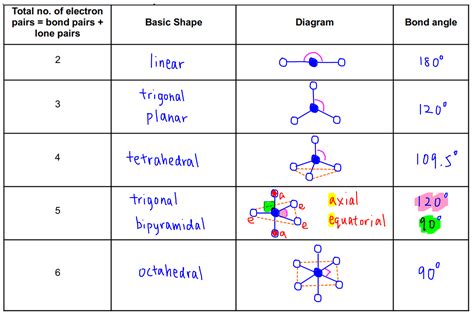
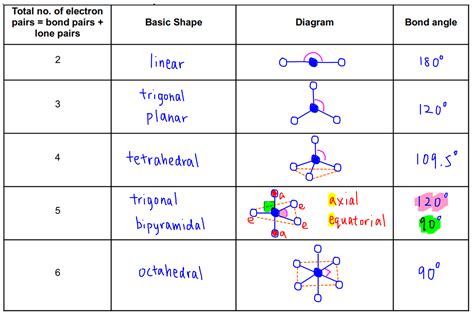
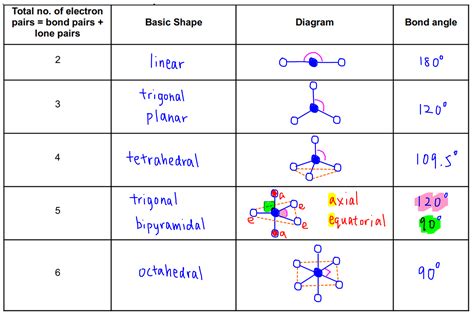
The VSEPR chart is a useful tool for visualizing and predicting the shape of molecules. It is a diagram that shows the arrangement of electron pairs around a central atom, allowing users to predict the shape of the molecule. The chart takes into account the number of electron pairs, lone pairs, and bonding pairs, providing a comprehensive understanding of the molecular structure. By using a VSEPR chart, users can quickly and easily determine the shape of a molecule, which is essential for understanding its chemical and physical properties.
Introduction to VSEPR Charts

VSEPR charts are an essential tool for chemists, researchers, and students. They provide a visual representation of the molecular structure, allowing users to quickly and easily determine the shape of a molecule. The chart is typically divided into sections, each representing a different type of electron pair. By using a VSEPR chart, users can predict the shape of a molecule, which is crucial for understanding its chemical and physical properties.
Benefits of Using VSEPR Charts
The benefits of using VSEPR charts are numerous. They provide a quick and easy way to predict the shape of a molecule, which is essential for understanding its chemical and physical properties. VSEPR charts also help users to visualize the molecular structure, making it easier to understand complex concepts. Additionally, VSEPR charts are a useful tool for identifying the polarity of a molecule, which is crucial for understanding its chemical properties.How to Use VSEPR Charts

Using a VSEPR chart is relatively straightforward. The first step is to determine the central atom of the molecule, which is typically the atom with the lowest electronegativity. Next, users need to determine the number of electron pairs, lone pairs, and bonding pairs around the central atom. This information is then used to determine the shape of the molecule, which is predicted using the VSEPR theory.
Steps to Determine the Shape of a Molecule
The steps to determine the shape of a molecule using a VSEPR chart are as follows: * Determine the central atom of the molecule * Determine the number of electron pairs, lone pairs, and bonding pairs around the central atom * Use the VSEPR theory to predict the shape of the molecule * Use the VSEPR chart to visualize the molecular structureTypes of VSEPR Charts
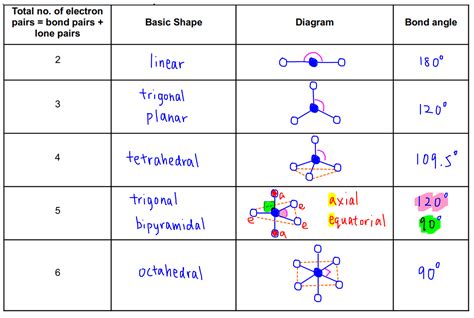
There are several types of VSEPR charts available, each with its own unique features and benefits. The most common type of VSEPR chart is the basic chart, which provides a simple and easy-to-use format for predicting the shape of molecules. Other types of VSEPR charts include the advanced chart, which provides more detailed information about the molecular structure, and the 3D chart, which provides a three-dimensional representation of the molecule.
Advantages and Disadvantages of Each Type
Each type of VSEPR chart has its own advantages and disadvantages. The basic chart is easy to use and provides a quick and simple way to predict the shape of molecules. However, it may not provide enough detail for more complex molecules. The advanced chart provides more detailed information about the molecular structure, but it can be more difficult to use. The 3D chart provides a three-dimensional representation of the molecule, which can be useful for visualizing complex molecular structures.Printable VSEPR Chart Templates

Printable VSEPR chart templates are available online, providing users with a convenient and easy-to-use format for predicting the shape of molecules. These templates can be downloaded and printed, allowing users to use them in the classroom or laboratory. The templates typically include a basic chart, as well as more advanced charts for predicting the shape of complex molecules.
How to Create a Printable VSEPR Chart Template
Creating a printable VSEPR chart template is relatively straightforward. The first step is to determine the type of chart that is needed, whether it is a basic chart or a more advanced chart. Next, users need to decide on the format of the chart, including the size and layout. The chart can then be created using a software program, such as Microsoft Word or Excel.Common Applications of VSEPR Charts
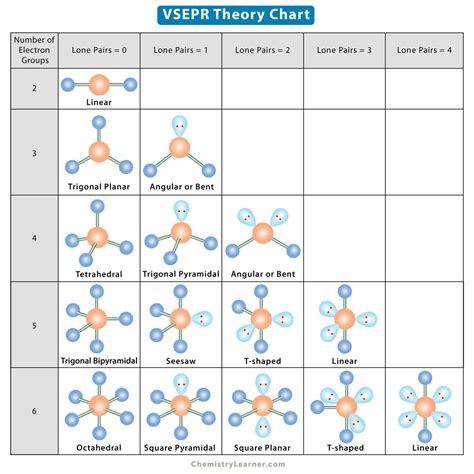
VSEPR charts have a wide range of applications in chemistry and other fields. They are commonly used in the classroom to teach students about the VSEPR theory and how to predict the shape of molecules. VSEPR charts are also used in research laboratories to predict the shape of complex molecules and to understand their chemical and physical properties.
Real-World Examples of VSEPR Charts in Action
VSEPR charts are used in a variety of real-world applications, including: * Predicting the shape of molecules in the pharmaceutical industry * Understanding the chemical properties of molecules in the petroleum industry * Predicting the shape of molecules in the field of materials scienceGallery of VSEPR Chart Examples
VSEPR Chart Image Gallery
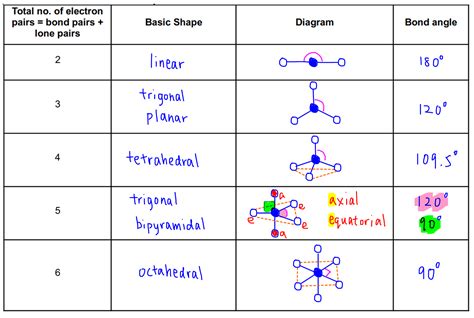
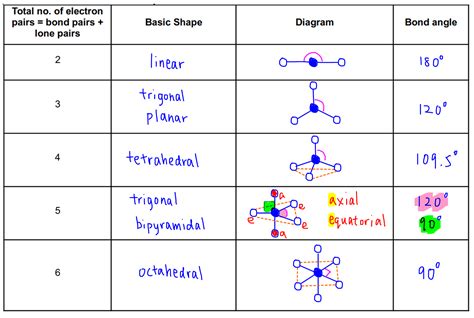



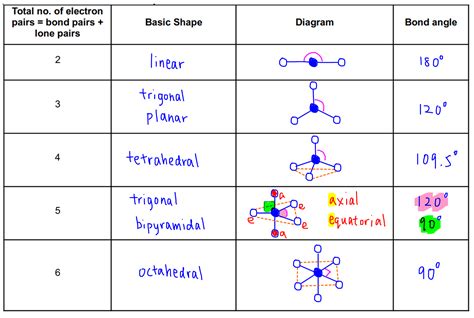
Frequently Asked Questions
What is the VSEPR theory?
+The VSEPR theory is a concept in chemistry that helps predict the shape of molecules. It states that electron pairs in the valence shell of an atom repel each other and arrange themselves to minimize repulsion.
How do I use a VSEPR chart?
+To use a VSEPR chart, determine the central atom of the molecule, determine the number of electron pairs, lone pairs, and bonding pairs around the central atom, and use the VSEPR theory to predict the shape of the molecule.
What are the benefits of using VSEPR charts?
+The benefits of using VSEPR charts include providing a quick and easy way to predict the shape of molecules, helping users to visualize the molecular structure, and identifying the polarity of a molecule.
Can I create my own VSEPR chart template?
+Yes, you can create your own VSEPR chart template using a software program such as Microsoft Word or Excel. Determine the type of chart you need, decide on the format, and create the chart.
What are some common applications of VSEPR charts?
+VSEPR charts have a wide range of applications in chemistry and other fields, including predicting the shape of molecules in the pharmaceutical industry, understanding the chemical properties of molecules in the petroleum industry, and predicting the shape of molecules in the field of materials science.
In conclusion, VSEPR charts are a valuable tool for predicting the shape of molecules and understanding their chemical and physical properties. By using a VSEPR chart, users can quickly and easily determine the shape of a molecule, which is essential for understanding its chemical and physical properties. We hope this article has provided you with a comprehensive understanding of VSEPR charts and their applications. If you have any further questions or would like to learn more about VSEPR charts, please don't hesitate to comment or share this article with others.
